Catalysis is an extremely important subfield of the chemical sciences which enables reactivities otherwise difficult to access. Computation can enable elucidation of the mechanism of action for catalysts and thereby lead to better design. Modeling catalysis is nonetheless challenging, entailing identification of key reactive channels out of many possibilities and simulation of unstable configurations. The nature of competing/inactivation pathways is also often of interest, to be able to obstruct them more efficiently.
Catalysis is often driven by transition metal species or enzymes. There is also growing interest in using external energy in the form of light (photocatalysis) or an applied potential (electrocatalysis). I have been interested in all of these areas, with some of my work noted below. The resulting publications are listed here.
Low-Coordination Iron Complexes
First-row transition-metals are increasingly of interest as catalysts, due to greater earth abundance than heavier elements like Rh or Ir. Coordinatively unsaturated metal complexes in particular can easily bind to substrates, and activate them via electron transfer processes. Working with experimental colleagues working in the group of Prof. T. Don Tilley, I explored the reactivity of low coordination iron(I) complexes for catalysis. We elucidated the catalytic mechanism for alkyne cyclotrimerization to arenes, and studied noncovalent interactions that stabilize low-coordination catalysts.
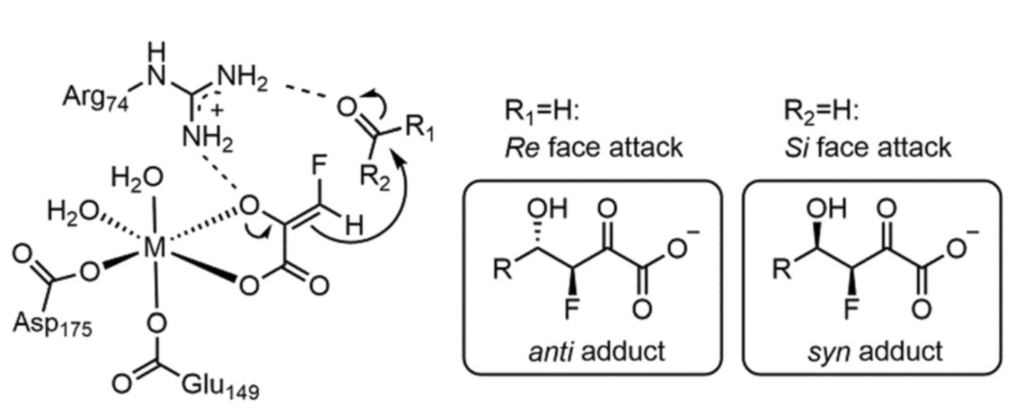
Asymmetric Biocatalysis
There is increasing interesting in harnessing the catalytic properties of enzymes for chemical synthesis. In particular, enzymes offer a straightforward route to chirality in organic molecules. It would thus be helpful to determine atomistic pathways leading to chiral selectivity at enzyme active sites. Working with experimental colleagues in Prof. Michelle Chang’s group, I helped elucidate the origins of chirality in synthesis of organofluorine compounds with aldolase enzymes. This could permit synthesis of organofluorine compounds with potential pharmaceutical utility.